Visually Clean: Bringing Clarity to Regulatory Guidelines
By Thomas Altmann, Global CIP/COP Technical Manager Cleaning Validation
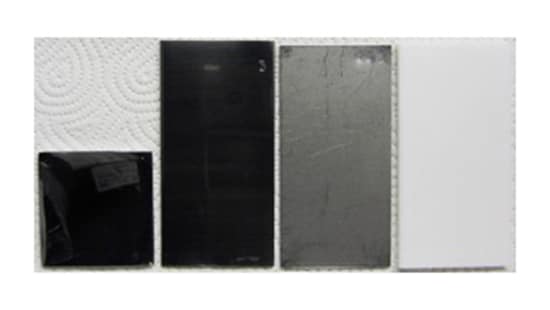
Visually clean is a term used in almost all pharma regulations (FDA, PIC/S, GMP). Simply stated, equipment used in pharmaceutical manufacturing has to be inspected for visual cleanliness prior to use1.
It makes perfect sense — but few are 100% clear on what a visual clean means in standard operating procedures and how to document it for cleaning validation. The most common questions include:
- What do residues look like?
- What is the visual threshold?
- How does the material of construction affect visibility?
- How often does an inspector’s eyesight have to be tested?
Below you’ll find a brief discussion of each of those issues.
What do residues look like?
Your inspectors need to know what they’re looking for when inspecting equipment. They should at best able to differentiate between process residues and residues left by detergents and sanitizing products — and how low levels of each type of residue would appear. Additionally, inspectors must have the knowledge regarding how the visual clean surface looks, including coloration of steels, scratches or other damages not influencing the next production step.
For example, Active Pharmaceutical Ingredients (API) often appear as powders and detergent residues can be seen as spots on the surface.

API residues often appear as powders.

Detergent residues often appear as spots.
Detergent residues are frequently missed to the “visual clean” inspectors. Pharma manufacturers using detergents designed for food and beverage applications should be aware that residues of non-water-soluble ingredients like corrosion inhibitors or specific surfactants may remain and create an unexpected interaction with residues of APIs and may be carried over into the subsequent manufactured product.
How does the material of construction affect appearance?
Residues may look different depending of the material used to construct the equipment being inspected. Roughly 90% of the equipment used in pharmaceutical manufacturing is made of stainless steel — with glass, rubber and polymers such as Teflon and EPDM accounting for most of the remainder.
When selecting equipment and training inspectors, one consideration should be that spotting residues on polymer surfaces will be more difficult than spotting residues on equipment made of stainless steel.
That said, stainless steel equipment exposed to high heat may/will discolor over time – making it more difficult to identify residues than when the equipment was delivered. Any equipment surfaces that become scratched will have to be re-polished (if possible) or replaced, once a visual clean can no longer be safely verified.

Inspectors must be trained to spot how each type of residue appears on each type of surface.

Residue appearance may be affected by different material finishes (stainless steel versus Teflon or new/polished versus discolored stainless steel.
What is the visual threshold?
The visual threshold is the concentration level when an API or residue becomes visible. Manufactures need to determine the visual threshold for each API or residue — and then compare it to the level when that API or residue becomes toxicologically significant. If the visual threshold is lower than your predetermined toxicologically significant level for that API or cleaning residue, then visual clean is a valid measure.
The analysis below shows the concentration at which Paracetamol (a relatively low-risk API) becomes visible.
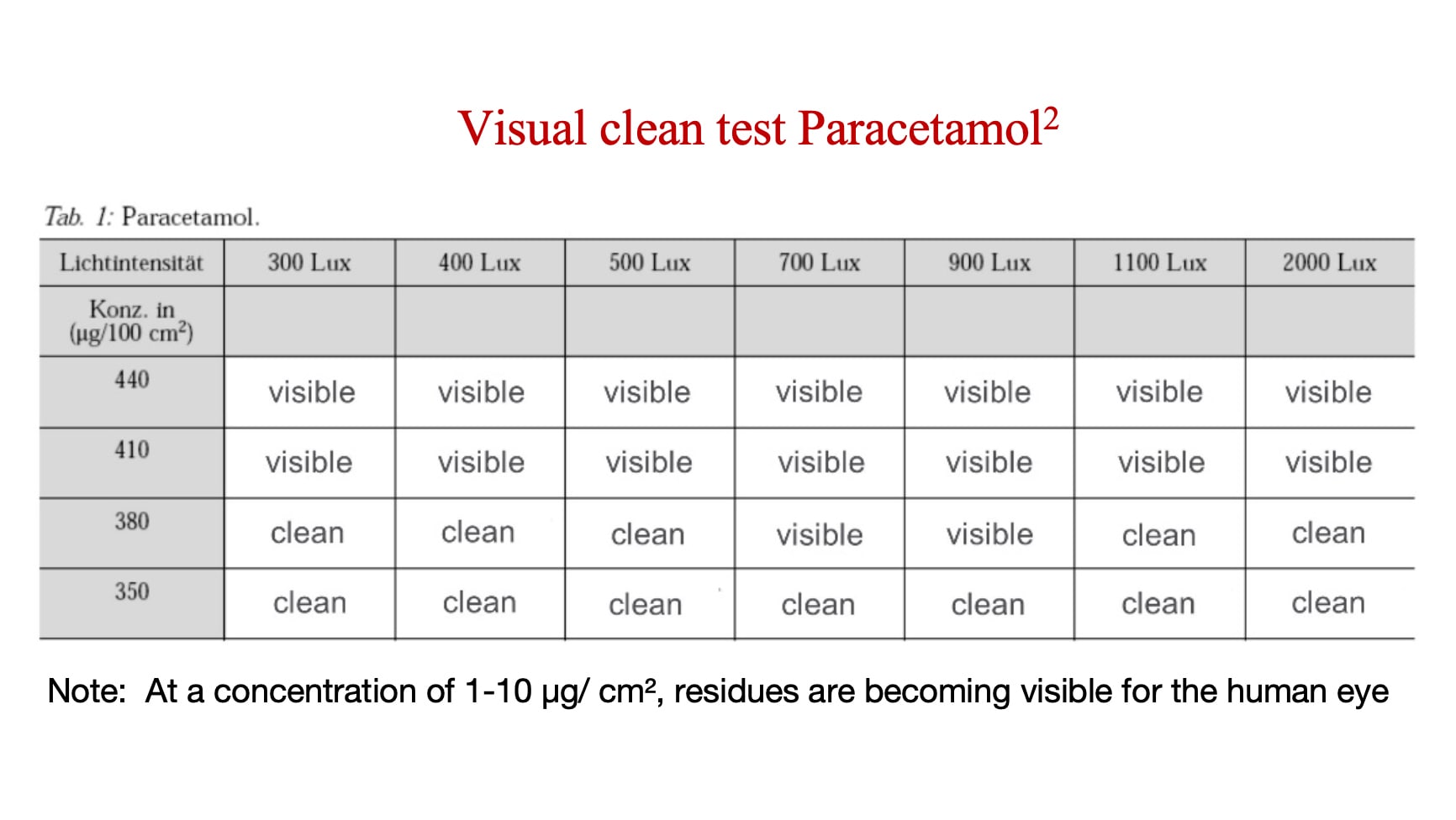
How often does an inspector’s eyesight have to be tested?
Once again, the guidelines are not specific. Many companies now require that all operators who are doing visual inspections have to have an eye exam every year.
It’s not required by law. On the other hand, if your cleaning procedures come into question, documenting that you’ve provided thorough training to your inspectors and then tested your inspectors vision on a regular basis will go a long way toward convincing regulatory agencies that you’ve done all that could be reasonably expected.
Additional questions?
For more information about best practices and visual clean guidelines, please contact Ecolab Life Sciences. We’re present on pharmaceutical sites around the world and can provide insights on how other manufacturers have implemented visual clean into their cleaning validation programs.
1FDA - 21 CFR Part 211.67 required in point (6) Inspection of equipment for cleanliness immediately before use
2Pharm. Ind. 62, Nr. 6 (2000) Buscalferri et al. − Reinigungsvalidierung


