A Review of Efficacy Testing of Disinfectants – The Relevance of a Wet Contact Time

Introduction
The use of disinfectants as agents to control microbiological contamination of an environment is well established and is governed by regulatory bodies in both Europe and the United States.
The Biocidal Products Regulation (BPR) 528/2012 [1] covers the marketing and use of biocidal products in Europe. Chemical disinfectants in the United States are registered and regulated by the U.S. Environmental Protection Agency (EPA) under Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) (40 CFR Parts 150-189) [2]. Under FIFRA, chemical disinfectants are considered “antimicrobial pesticides”.
Data demonstrating the efficacy claim of a disinfectant, whether it is bactericidal, fungicidal, sporicidal or viricidal, is a clear requirement of BPR or EPA for a disinfectant manufacturer to achieve registration.
For disinfectant end-users within the pharmaceutical sector, regulations also state the need for them to demonstrate disinfectant efficacy. The US Food and Drug Administration (FDA) guidance for pharmaceutical industry states “The suitability, efficacy, and limitations of disinfecting agents and procedures should be assessed. The effectiveness of these disinfectants and procedures should be measured by their ability to ensure that potential contaminants are adequately removed from surfaces” [3]. Within Europe similar requirements are provided by the European Commission, “4.37 The disinfection process should be validated. Validation studies should demonstrate the suitability and effectiveness of disinfectants in the specific manner in which they are used and should support the in-use expiry periods of prepared solutions.” [4].
Methods to Demonstrate Efficacy
In Europe, European Norm (EN) standard 14885:2018 [5] provides references to required test methods (EN standards) to be used by disinfectant manufacturers to support claims of microbiocidal activity. In the United States, EPA Product Performance Test Guideline OCSPP 810.2100 [6] details the test methods (Association of Official Analytical Chemists (AOAC) methods) to be used by disinfectant manufacturers to support claims of microbiocidal activity.
The test types can be split into two categories:
- Suspension Testing
e.g. EN 1276 Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas. Test method and requirements (phase 2, step 1).
EN 1650 Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of fungicidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas. Test method and requirements (phase 2, step 1).
AOAC Official Method 955.15 Testing Disinfectants Against Staphylococcus aureus, Use-Dilution Method.
- Surface Testing
e.g. EN 13697 Chemical disinfectants and antiseptics – Quantitative non-porous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas – Test method and requirements without mechanical action (phase 2, step 2).
AOAC Official Method 961.02 Germicidal Spray Products as Disinfectants.
The different test methods use specific starting inoculum, organisms, log reductions and contact times.
For making disinfectant claims within Europe, disinfectant manufacturers usually perform the EN tests, and the EN test numbers may be provided on the product labels, along with the standard contact times specified in the method. The standard test methods are usually used as they are robust, reproducible and well recognized. It can also be useful for end users to be able to refer to results from standard method testing to enable them to compare products from different manufacturers.
For an end user of a disinfectant, the standard test methods may not accurately reflect the conditions in their own pharmaceutical cleanroom. End users will typically have different surface materials in their cleanrooms, different microorganisms present and different environmental conditions (such as low humidity, rapid drying due to HVAC systems).
The United States Pharmacopoeia (USP) chapter <1072> [7] provides further advice on efficacy testing for the pharmaceutical sector, stating “To demonstrate the efficacy of a disinfectant within a pharmaceutical manufacturing environment, it may be deemed necessary to conduct the following tests: (1) use dilution tests (screening disinfectants for their efficacy at various concentrations and contact times against a wide range of standard test organisms and environmental isolates); (2) Disinfectant surface challenge tests (using standard test microorganisms and microorganisms that are typical environmental isolates, applying disinfectants to surfaces at the selected use concentration with a specified contact time, and determining the log reduction of the challenge microorganisms); and (3) a statistical comparison of the frequency of isolation of microorganisms isolated prior to and after the implementation of a new disinfectant.
This is considered necessary because critical process steps like disinfection of aseptic processing areas, as required by GMP regulations, need to be validated, and the EPA registration requirements do not address how disinfectants are actually used in the pharmaceutical, biotechnological, and medical device industries.
In line with the USP guidance it is relatively easy to use different microorganisms and test surfaces with standard test methods. However, achieving the specified contact times of the standard test methods can be challenging within the environmental conditions of a pharmaceutical cleanroom. The evaporation rate of a disinfectant wiped onto a surface in a cleanroom with a high air change rate could be significantly different to evaporation rate under laboratory conditions. This raises questions as to whether the surface must be visibly wetted with disinfectant for the specified length of time to achieve efficacy, and therefore what exactly the expectation of a contact time is.
Definitions of Contact Time
In order to establish if it is a pharmaceutical sector regulatory requirement for surfaces to remain visibly wet for the duration of contact time (hereafter referred to as “wet contact time”), a review of regulations was undertaken.
US [3],[8], EU GMP [4] and Pharmaceutical Inspection Co-operation Scheme [9] guidelines do not define ‘contact time’. The European Pharmacopoeia (EP) does not provide any guidance on disinfectant efficacy testing. In contrast, the USP Chapter 1072 [7] uses the term ‘contact time’ frequently but does not detail its meaning in the definitions section of the chapter. The ISO standard for cleanrooms (ISO 14644 part 5 [10]) also does not provide any definition.
As the pharmaceutical sector regulations do not provide a clear meaning, a wider search of other applicable regulatory documents and recognised pharmaceutical industry guidance was performed.
Royal Pharmaceutical Society/NHS Pharmaceutical Quality Assurance Committee provides guidance on the application of disinfectants, stating “Pooling of excess amounts of cleaning and disinfecting agents should be avoided. Ideally surfaces should become dry within 1 hour of application. Conversely, sufficient product should remain to achieve the required efficacy throughout the recommended contact time i.e. disinfectants should not be spread too thinly” [11]. This statement infers an expectation that a wet contact time is required to achieve efficacy.
The EN and AOAC methods do not specify a ‘wet contact time’. In suspension tests, a ‘wet contact time’ is always used as the test involves addition of the disinfectant product to an organism suspension held in solution for the required contact time, with product neutraliser added at the end of this contact time.
It is not as clear for surface tests, however, as an amount of disinfectant, as defined by the standard, is pipetted onto surface without spreading it out. Because of the small volumes that are pipetted, and the relatively high surface tension of most disinfectants tested, it is likely that a wet contact time will be achieved under laboratory conditions.
The EN surface test with mechanical action, EN 16615 [12], provides the test method closest to the practical use of disinfectants because it incorporates the action of wiping the disinfectant onto a surface. Under the Experimental Conditions section, the following information is provided regarding contact time: “The contact times for surface disinfectants are chosen on the basis of the practical conditions of the product. The recommended contact time for the use of the product is within the responsibility of the manufacturer.” It could be inferred from the wording “practical conditions of the product” that they are referring to volumes applied by mopping or wiping and evaporation rates, for example an alcohol compared to a quaternary ammonium compound, but again this is not a clear definition.
The Pharmaceutical and Healthcare Sciences Society (PHSS) Technical Monograph 20 provides the following information under section 3.3.4 “The coupons are exposed to disinfectant for the defined wet / residence contact time” [13]. This is a clear statement that the contact time is wet.
The US Center for Disease Control and Prevention (CDC) Guideline [14] provides the following glossary definition of contact time: “Time a disinfectant is in direct contact with the surface or item to be disinfected. For surface disinfection, this period is framed by the application to the surface until complete drying has occurred.” This definition is also clearly stating a wet contact time.
Product Performance Test Guideline, OCSPP 810.2000 [15], provides the following information on contact time: “The contact time used in efficacy testing should be the same or shorter than the contact time identified on the product label. If a contact time is different from the range identified in the test method or guideline is preferred, consultation with the agency prior to testing is recommended and a modification to the standard approach may be needed. In most cases, a modification to provide a longer exposure period is limited by the practical considerations of the use patterns (e.g., an exposure period of >10 min for a product that will likely evaporate from the treated surface within 10 min). Clearly identify and justify all method modifications in the test protocol. For liquid or spray products containing volatile active ingredients where the product is applied to a hard non-porous surface, the maximum contact time may be determined by visually inspecting evaporation over the proposed contact period.” Once again, this statement clearly indicates an expectation for a wet contact time.
PDA technical report 70 [16] provides the following glossary definition: “The minimum amount of time that a sanitizer, disinfectant, or sporicide must be left in complete (wet) contact with the surface to be treated in order to be effective”. This definition is clearly stating a wet contact time.
Pharmig’s Guide for Disinfectant Use [17] gives the following text: “More rapid evaporation of the disinfectant may occur on warm surfaces or where the treated surface is subject to low humidity or high airflow conditions, as is sometimes found in cleanroom operations.” This statement infers an expectation that a wet contact time is required to achieve efficacy.
From the above review of respected pharmaceutical industry publications, the need for contact time to be wet is clearer.
It is not inconceivable that there is a continuation of disinfectant efficacy after the surface is visibly dry as the action is taking place at a cellular level. The first stage of microbial kill is the uptake of the active ingredient in the disinfectant by the cell. It can therefore be considered that there are two “times” in play during disinfection (Figure 1).
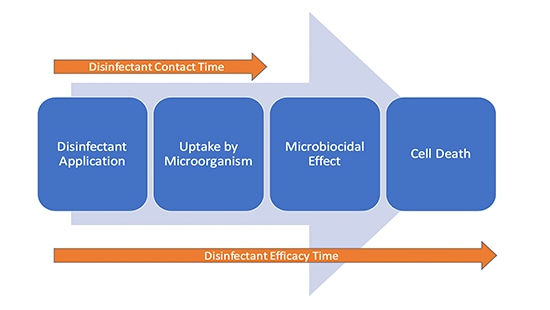
Conclusion
To demonstrate efficacy a disinfectant supplier is required to execute standard tests under repeatable conditions, from which they will define a contact time. This contact time might prove useful to the end user in selection of the appropriate disinfectant.
An end user must also validate disinfectant efficacy, reflecting the conditions of use within their facility including defining a contact time used in practice.
Most pharmaceutical guidance organizations define contact time as a wet contact time.
There are only limited studies published to investigate disinfectant performance after surfaces were visibly dry. It is also impossible to measure in practice as the user cannot observe cell death as an endpoint.
To facilitate end user testing that is representative of their facility conditions, they are encouraged to measure the time taken for disinfectants to evaporate when applied using routine techniques (wiping/mopping) and use this contact time for laboratory studies.
References:
- Regulation (Eu) no. 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocidal Products
- Title 40 of the Code of Federal Regulations (40 CFR) Subchapter E Pesticide Programs Parts 150-189
- FDA Guidance for Industry - Sterile Drug Products Produced by Aseptic Processing, Current Good Manufacturing Practice (2004)
- EudraLex Volume 4 EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use Annex 1 Manufacture of Sterile Medicinal Products (Draft v.12, issued February 2020)
- EN 14885:2018 Chemical Disinfectants and Antiseptics. Application of European Standards For Chemical Disinfectants and Antiseptics
- EPA Product Performance Test Guideline, OCSPP 810.2100, Sterilants, Sporicides, and Decontaminants, Guidance for Efficacy Testing, [EPA 712-C-17-003]
- United States Pharmacopoeia (USP) chapter 1072 Disinfectants and Antiseptics
- The Code of Federal Regulations CFR Title 21 - Food and Drugs: Parts 1 to 1499
- PICS GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS ANNEXES PE 009-14
- ISO 14644 Part 5 Cleanrooms and associate controlled environments - Part 5: Operations, 2004
- Royal Pharmaceutical Society. Quality Assurance of Aseptic Preparation Services: Standards. Part A 5th Edition 2016
- EN 16615 Chemical disinfectants and antiseptics – Quantitative test method for the evaluation of bactericidal and yeasticidal activity on non-porous surfaces with mechanical action employing wipes in the medical area (4-field test) – Test method and requirements (phase 2, step 2)
- Pharmaceutical and Healthcare Sciences Society (PHSS) Technical Monograph 20 Bio-Contamination characterisation, control, monitoring and deviation management in controlled / GMP classified areas
- Center for Disease Control and Prevention (CDC) Guideline for Disinfection and Sterilization in Healthcare Facilities (2008)
- Product Performance Test Guideline, OCSPP 810.2000, General Considerations for Testing Public Health Antimicrobial Pesticides, Guidance for Efficacy Testing, [EPA 712-C-17-002]
- Parenteral Drug Association (PDA) Technical Report No. 70. The Fundamentals of Cleaning and Disinfection Programs for Aseptic Manufacturing Facilities (2015).
- Pharmig Guide to Disinfectants and their use in the Pharmaceutical Industry (2017)
- West AM, Teska, PJ, Oliver HF; There is no additional bactericidal efficacy of Environmental Protection Agency registered disinfectant towelettes after surface drying or beyond label contact time. Am J Infect Control 2019; 47: 27-32



