
CLEEN by Ecolab:
Digitally Enhanced
Manufacturing Operations
Reduce Compliance Risks. Drive Quality. Optimize Operations.
What Is CLEEN?
CLEEN by Ecolab is a software platform that enables pharmaceutical and personal care organizations to drive improvement across the entire manufacturing lifecycle—from automating cleaning validation and digitizing SOPs to protecting data integrity and driving operational efficiencies in batch manufacturing. The end-to-end CLEEN solution combines Ecolab expertise with a best-in-class digital platform purpose-built for manufacturing support.

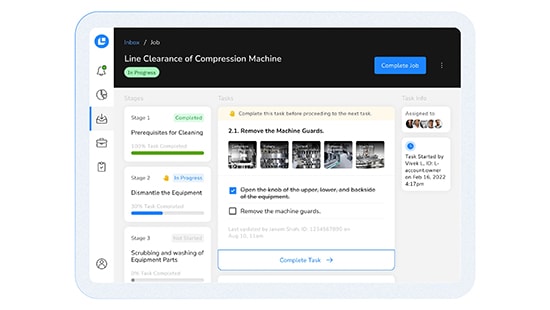
CLEEN Batch Execution Module
Transform batch record execution with enhanced efficiency, accuracy, and collaboration
CLEEN Digital Logbook Module
Digitize SOPs to drive performance and compliance.
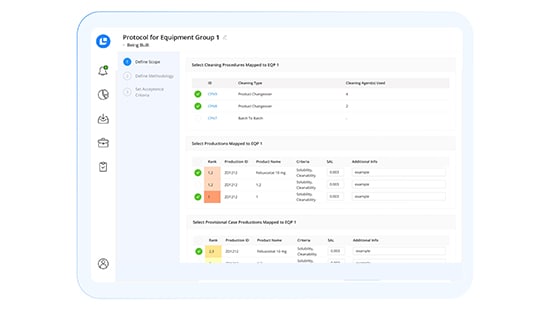
CLEEN Validation Module
Modernize cleaning validation with intelligent automation.

See how Valent BioSciences transformed manual record-keeping into data-driven operational efficiency with CLEEN.
Ecolab has provided chemistry solutions to Valent BioSciences for nearly a decade, and the same trusted partnership came through in Valent BioSciences experience with the CLEEN platform.
Benefits of CLEEN by Ecolab
Reduce Compliance Risk
Strengthen your cleaning validation program with intelligent recommendations and guidance around regulatory requirements.
Implement digitized process interlocks and provide real-time insights into operator actions, along with a complete audit trail. Continuous monitoring of critical process parameters provides 24/7 audit readiness.
Drive Quality
Digitize workflows and work instructions and leverage real-time analytics to identify inefficiencies and opportunities for improvement.
Automate cleaning validation protocols and reduce changeover times while lowering costs and reallocating labor to other value-add tasks.
Increase Top-line Revenue
Accelerate changeover times and drive meaningful gains in production capacity.
Provide real-time tracking, scheduling, and resource allocation leading to reduced downtime, higher throughput, and maximized utilization of equipment and labor.
Out-of-the-Box Data Connections for Rapid Integrations
ERP | QMS | LMS | DMS | SSO | EM
Security Certifications and Compliance
Compliant and Scalable Cloud Infrastructure
GxP Compliant, ISO 27001 Certified
Data Centers
- Data centers are certified by 3rd party auditors for ISO 9001:2015, ISO 27001
- GxP guidelines available and implemented
SSO and LDAP
- Single sign-on (SSO) and lightweight directory access protocol (LDAP) allow you to authenticate users in systems
- Configurable by customer IT team
Vulnerability Assessment and
Penetration Testing
- Periodic VAPT
- Performed by certified 3rd party
- Results shared with customers on-demand
End-to-End Data Encryption
for Virtual Machines
- Server side encryption with PMK
- Data sent to or from Leucine is encrypted in transit using 256 bit encryption
Domain Whitelisting
- Allow or disallow domains that can access the platform
- Configurable by customer IT team

Backup and Disaster Recovery
- Daily backups available
- Disaster recovery Regions
- Complete and incremental backups

Embracing Pharma 4.0
Why operational digitization must be the top priority for pharma and biopharma manufacturers.
This free eBook outlines the fundamental shifts associated with embracing Pharma 4.0—and how they can empower organizations to improve efficiency, agility, and maintain product quality while driving business profitability.

Avoiding Top FDA Form 483 Observations in Pharma Manufacturing
Why proactive measures must be the top priority for pharma manufacturers.
This comprehensive article outlines the critical observations that can be avoided in pharma manufacturing and how taking proactive measures can enhance compliance, ensure product quality, and maintain regulatory standards while optimizing operational efficiency.


